SARomics Biostructures 2018: A Foundation For the Future
It was a fantastic year for the company! We introduced new team members and new services, including WAC fragment screening…
It was a fantastic year for the company! We introduced new team members and new services, including WAC fragment screening and characterization of the higher-order structure of biosimilars, and many new customers found their way to us!
SARomics Biostructures entered an expansive phase, and things started to move quickly. Last year may be described by one single word – fantastic! The company employed four new people, introduced new services, and many new customers found their way to us.
Perhaps one of the most exciting moments was that our newly introduced weak affinity chromatography (WAC) fragment library screening services started to give results – with the introduction of WAC, our integrated drug discovery platform was “discovered” by big pharma as well as smaller biotech companies. We believe that the new screening technology, in combination with the extensive biophysical competence at SARomics Biostructures, will pave the way for us to attack certain classes of drug targets with high innovative potential, notoriously known for the difficulties associated with the identification of new hits/inhibitors against them. Together with our close partner, Red Glead Discovery, we now have a total capacity to run fragment-to-lead or gene-to-lead projects successfully. Our screening capabilities using biophysical methods, like X-ray crystallography, protein NMR spectroscopy and other techniques, are highly complementary to WAC and may be used, e.g., for the characterization of the binding site and detailed analysis of protein-ligand interactions.
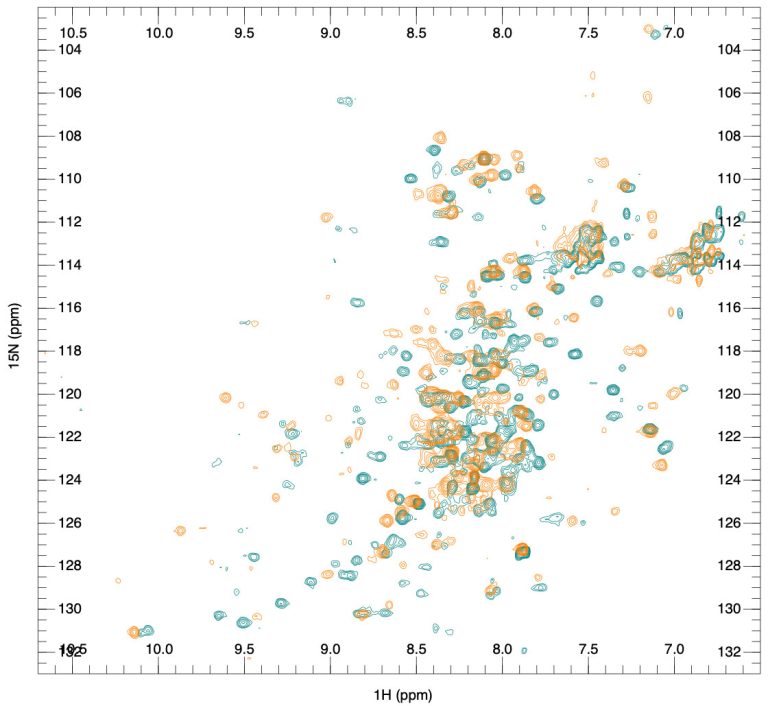
Introduction of biosimilar testing services, including higher-order structure characterization – these services are based on recent advances in NMR spectroscopy that have made it possible to acquire a unique fingerprint representation of the 3D conformation of large molecules like biologics without expensive isotope labeling. By directly matching the NMR fingerprint of a given protein to its high-resolution three-dimensional structure, we can rapidly assess the comparability of a biosimilar and its originator or different batches of the same biologic. These services are of high value for customers since FDA registration of biosimilars is much more complicated and more expensive than registration of generic small molecule drugs and requires more advanced product characterization. With the biologics/biosimilars market’s current growth, we believe these services will see a considerable increase in the coming years.
In addition to the biosimilar testing services, we have “officially” introduced the co-crystallization of antigen-antibody complexes. Although we have been doing this for a while and accumulated extensive experience within the area, we never had a separate page dedicated to these services on our website. We now have a separate description of these services for our clients for simplicity and convenience.
Our most popular crystallography services pipeline has also been upgraded: Our off-the-shelf FastLane™ Premium and FastLane™ standard libraries have been complemented with several new structures.
Often, the crystallography services pipeline is used to co-crystallize the protein in question with the client’s ligand (or a set of ligands). In the case of gene-to-structure projects, we clone, express, purify and crystallize the protein. In the case of FastLane Premium structures, we usually have the protein stored in the lab, and crystallization drops can be set up immediately after the ligands’ arrival. We have construct and verified protocols for the expression, purification, and crystallization of the proteins from the FastLane Standard library. In some cases, it has taken us only about two weeks from signing the contract with the customer to the three-dimensional structure of the protein complex.
It is well known that the CRO market is in a state of constant expansion. Most small-to-mid-size biotech companies typically lack structural biology capabilities and often even biophysical competence since core competencies at these companies are generally focused on biochemistry and molecular biology. SARomics Biostructures’ technology platform is well-prepared to meet these needs by providing reliable data at the best quality level.